Overview of Stem Cell Therapy
Stem Cell Therapy Ltd. (tentative name), established in the 2010s, is a biotechnology firm headquartered in Seoul, with a GMP (Good Manufacturing Practice) facility located in Gyeonggi Province. The company employs a dedicated team of 42 professionals. Stem Cell Therapy Ltd. has carved a niche in the biotechnology industry by focusing on the development of cell-based therapies for bone diseases and cancer immunotherapy. Alongside its primary operations, Stem Cell Therapy Ltd. engages in ancillary businesses such as the provision of cells, media, CMO (Contract Manufacturing Organisation) services, and analytical services to support the broader biotechnology sector.
Business of Stem Cell Therapy
Stem Cell Therapy Ltd.’s core business revolves around the research, development, and commercialisation of allogeneic cell therapies for treating bone disorders and autologous immune cell therapies for cancer. The company has prioritised the advancement of platform technologies, including the refinement of hydrogel 3D cultures and a specific technique for cultivating and differentiating cells aimed at treating bone diseases. This focus extends to developing a robust pipeline for cartilage, ligament, muscle, and cancer therapies, emphasising the scalability of technology from laboratory to industrial application. Stem Cell Therapy Ltd. is committed to ensuring the mass production of advanced biopharmaceuticals through the establishment of GMP facilities and the acquisition of IP for critical process and preservation technologies.
The company’s motivation stems from addressing unmet needs in the market, particularly in conditions ranging from osteonecrosis to spinal fusion, aiming to expand its market through the broad application of cell therapies and the enhancement of biomanufacturing capabilities. From its inception through to the current day, Stem Cell Therapy Ltd. has achieved significant milestones, including clinical trial approvals, patent registrations, and the attainment of ISO9001 certification, demonstrating its commitment to innovation and quality in biomedicine.

Technologies of Stem Cell Therapy
Stem Cell Therapy Ltd. has developed a suite of technological platforms centred on advanced cell engineering for therapeutic purposes. The company’s technology can be broadly categorised into two main areas.
- 3D Cell Culture Technology: This involves cultivating cells in a 3D environment, which better simulates the conditions within the human body compared to traditional two-dimensional (2D) culture systems. This method is crucial for producing cells that are more physiologically relevant and, therefore, potentially more effective in treating various conditions.
- Hydrogel-Based Cell Cultivation: Hydrogels are used as a scaffold within which cells can grow and develop in three dimensions. This mimics the extracellular matrix found in the body, providing structural support and enabling cells to maintain their shape and function.
The combination of these technologies allows Stem Cell Therapy Ltd. to develop ‘off-the-shelf’ cell therapy products that are ready to use. These products are designed to be easily integrated into existing medical practices and are GMP-compatible, meaning they meet the stringent quality requirements for clinical use. This technology is applied across a range of therapeutic areas, including bone and cartilage regeneration, which addresses significant unmet medical needs.
1. Three-Dimensional (3D) Cell Culture Technology
Stem Cell Therapy Ltd. has adopted 3D Cell Culture Technology as a cornerstone of its research and development activities. This technology represents a significant advancement from the traditional two-dimensional (2D) cell culture, where cells are grown in flat layers on a plate. In contrast, 3D cell culture allows cells to grow in all directions, akin to how they would in a living organism.
The 3D approach provides a more realistic environment for cells to interact, which is essential for understanding how they behave in the human body. This method has become increasingly important in the study of complex biological processes, drug development, and regenerative medicine.
In practice, 3D cell cultures are facilitated by the use of hydrogels, which serve as a scaffold to support the cells. These hydrogels are designed to mimic the natural extracellular matrix, providing the cells with the necessary cues for growth and function. By adjusting the stiffness of the hydrogels, a property known as ‘biomimetic tuning’, Stem Cell Therapy Ltd. can tailor the environment to suit different types of cells, from softer gels for bone or fat tissue cells to stiffer ones for bone or cartilage cells.
Furthermore, the company utilises ‘Designer Gels’, a type of tunable hydrogel, for creating conditions that guide stem cells along specific paths of development. This is crucial for stem cell therapy, where the aim is to produce cells that can replace damaged tissues in diseases or injuries.
2. Hydrogel-Based Cell Cultivation
Hydrogel-based cell cultivation is a sophisticated technique utilised by Stem Cell Therapy Ltd. to support the growth and development of cells in a three-dimensional environment. Hydrogels are water-rich polymers that can simulate the physical properties of a natural extracellular matrix, providing a supportive scaffold for cells. This environment allows cells to maintain their shape, proliferate, and differentiate in a manner more reflective of their natural state within the human body.
In the context of stem cell therapy, where the accurate differentiation of stem cells is crucial, the stiffness (or biomimetic tuning) of the hydrogel can be adjusted to mimic the conditions of different types of body tissues. For instance, softer hydrogels may be used to culture cells that will become bone tissue, while stiffer hydrogels are suitable for bone tissue cells. This precision in controlling the physical milieu of the cells is pivotal for guiding their development into the desired cell types.
Stem Cell Therapy Ltd.’s hydrogel technology is not only biomimetic but also tunable, allowing the manipulation of physical properties to direct stem cell lineage specification. This means that by altering the stiffness of the hydrogel, stem cells can be coaxed into differentiating into specific cell types required for repairing tissues or treating diseases.
The company’s approach includes the use of ‘Designer Gels’, which are hydrogels with finely tuned properties that can be adjusted for optimal cell growth and differentiation. These gels provide the necessary signals that influence cell behaviour, such as promoting bone formation or vascularisation in tissue engineering applications.
Stem Cell Therapy Ltd.’s hydrogel-based cultivation process is meticulously designed to support the 3D culture of cells. This process begins with the preparation of the hydrogel substrate, followed by the seeding of cells onto this substrate. The hydrogel provides a supportive and nutrient-rich environment, much like the natural extracellular matrix, allowing cells to grow and proliferate effectively.
After seeding, the cells are incubated within this hydrogel matrix, where they can expand in a three-dimensional space. This is a critical step, as it allows for the establishment of a robust cell culture that more accurately reflects the natural growth patterns and interactions of cells in living tissues.
The next phase involves the differentiation of these cells, a process which is guided by the change of media to one that is specific to the desired cell type. This media provides the biochemical cues necessary for the cells to differentiate. Differentiation is a highly controlled process that is essential for the production of cells with specific functions, such as bone, cartilage, or nerve cells.
An important aspect of Stem Cell Therapy Ltd.’s process is the use of gas-permeable films within the culture system. This feature ensures that the cells receive adequate levels of oxygen and that carbon dioxide is removed, mimicking the gas exchange that occurs in blood vessels. This gas exchange is essential for maintaining the cells’ metabolic processes and overall health.
Moreover, the company’s approach to hydrogel-based cell cultivation aims to optimise the physical space around the cells. The space must be sufficient to allow for cell expansion and growth but also needs to be regulated to prevent the formation of bubbles that could disrupt the uniformity of the cell culture.
By controlling these environmental parameters, Stem Cell Therapy Ltd. can cultivate cells that not only grow in a more physiologically relevant configuration but also maintain their function after being thawed from frozen states. This is particularly important for the development of off-the-shelf cell therapy products, which require a high degree of viability and efficacy post-thaw to be clinically useful.
The company’s cell cultivation method is demonstrated to be effective in producing cells that show homogeneity in growth and function, which is critical for ensuring the quality and consistency of therapeutic cell batches. This uniformity is vital when scaling up production for commercialisation and clinical application, ensuring that patients receive a reliable and effective cell-based therapy.
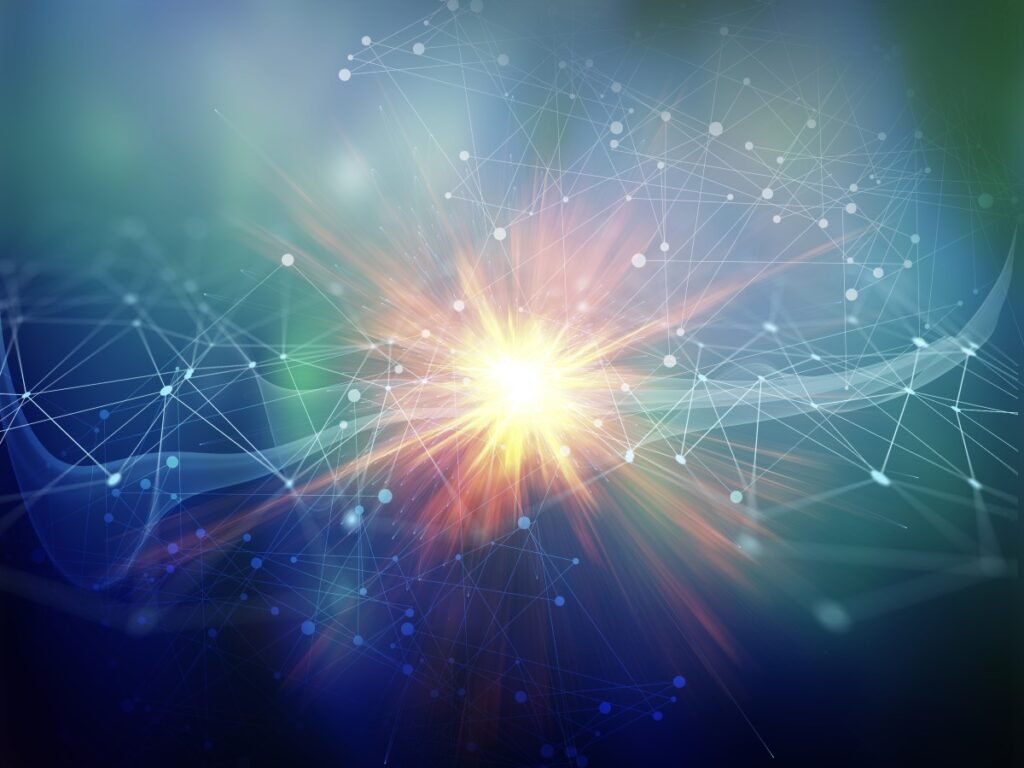
3. Evaluating Immunogenicity in Cell Therapy Development
When developing cell therapies, a critical factor to consider is the immunogenicity of the therapeutic cells. This refers to the potential of these cells to provoke an immune response in the recipient. The goal is to ensure that the cells will be compatible and not rejected by the patient’s immune system. For instance, experiments using a xenogeneic model, where human cells are implanted into non-human hosts, help determine whether the cells can survive without provoking an immune response that would lead to their rejection.
In vitro studies, such as the Mixed Lymphocyte Reaction (MLR), measure the proliferation of lymphocytes when exposed to the MSCs, providing insights into the level of immune response the therapeutic cells may induce. A low level of lymphocyte proliferation suggests that the cells are less likely to be rejected.
Further analysis in syngeneic models, where the donor and recipient are genetically identical, such as inbred rats or mice, provides additional data on the immune compatibility of the cells. This is crucial for autologous therapies, where the patient’s own cells are used for treatment.
In allogeneic therapies, where the cells are derived from a donor different from the recipient, it is essential to ensure that the donor cells do not carry high levels of immunogenic markers. The presence of such markers could lead to an adverse immune response in the recipient.
Ensuring low immunogenicity is a step towards achieving therapies with higher success rates and lower risks of complications related to immune rejection.
Company’s Products (Pipelines)
The company has developed a portfolio of medical products aimed at addressing various health conditions and diseases.
1. Osteonecrosis of Femoral Head
This condition involves the death of bone tissue due to a lack of blood supply and is being targeted by the product M801. The company has finished phase 1 of clinical trials, with a phase 2 Investigational New Drug (IND) application submitted. This condition typically affects patients around the age of over 36 and can result from complications arising from the use of steroids, excessive alcohol consumption, trauma, and certain medical conditions.
2. Hip Fragility Fracture
M801 is also being developed to address hip fragility fractures, often occurring in individuals over 65 years old, with a significant percentage being women. It’s noted that this condition has a high one-year mortality rate of 20-30% due to complications. The product is currently in phase 2 IND status.
3. Spinal Fusion
The product is designed for spinal fusion procedures, with a median patient age of 62.9 years. The product aims to improve outcomes in interbody cage surgeries, a common treatment for spinal instability. M801 is again the product in focus, with IND application status indicating ongoing clinical development.
4. Atypical Femoral Fracture
Targeting atypical femoral fractures, often a side effect of long-term bisphosphonate therapy used for osteoporosis, M801 is presented as a potential solution. The product is under clinical development, with a phase 2 IND application in place.
5. Solid Cancer
The company is addressing solid cancer with a product named DiNK. It’s being developed to improve survival rates in patients, as current five-year survival stands at 37.6%. The treatment is designed for advanced-stage cancer, with a focus on enhancing regional chemotherapy and combined hepatic artery infusion (HAIC). The approach is innovative, with the potential to impact survival in a disease affecting a significant patient population.
Market Prospect
The market for osteonecrosis of the femoral head is part of a larger sector that includes various orthopaedic conditions requiring surgical intervention. Although specific market size and growth rate data for osteonecrosis of the femoral head are not provided in the searched resources, the related hip replacement market is expected to grow at a CAGR of 5.46% between 2023 and 2028. This growth is driven by the increasing need for hip replacement surgeries due to conditions like osteoarthritis, rheumatoid arthritis, or fractures, which compromise the hip joint’s capability. The rise in demand for hip replacement procedures correlates with the ageing population and the persistence of musculoskeletal disorders. Advanced surgical techniques and materials for prosthetic implants contribute to successful outcomes, which is a significant factor in the market’s growth. The market value is anticipated to increase by around US$ 9 Billion by 2028.
For hip fragility fractures, the larger market context includes fracture fixation products, which are essential in the treatment and management of fractures. The global fracture fixation products market is expected to reach $15.2 billion by 2029, reflecting the critical need for these products in treating various fracture types. The internal fixation devices for large bones and intramedullary nail segments are particularly relevant for hip fractures, indicating significant market shares and anticipated fast growth. Geographically, North America and Asia Pacific are key regions, with Asia Pacific expected to see a rise in hip fractures as its elderly population grows.
In the case of spinal fusion, the market dynamics are influenced by the demand for procedures that stabilise the spine and relieve pain associated with spinal disorders. While specific figures for the spinal fusion market were not obtained from the provided resources, it is a part of the broader orthopaedic devices market. This market includes products like interbody cages used in spinal fusion surgeries, which are expected to see sustained development due to technological advancements and an increasing focus on minimally invasive surgical techniques.
Overall, these markets are part of a broader trend towards addressing ageing-related degenerative conditions and improving the quality of life for patients with musculoskeletal disorders. The anticipated growth in these markets is likely due to factors such as the ageing population, advancements in surgical procedures, and the development of new materials and technologies for orthopaedic implants.
Company’s Performance
The financial performance of the company reveals an increase in sales revenue over the last four years, mainly from its cosmetic ingredients sector as its non-core business. The figures show a positive trajectory from 2020’s revenue of approximately KRW 527 million to a projection surpassing KRW 1.37 billion in 2023. This consistent growth indicates a robust market presence and operational success in the cosmetics domain.
When considering the company’s core biotechnology pursuits, while no specific revenue figures are projected, the potential for market entry post-phase 2 clinical trials, estimated to be 3-4 years from now, is significant. This aligns with the broader industry trend where the orthopaedic device market, encompassing conditions like osteonecrosis of the femoral head, hip fragility fractures, and spinal fusion, is on a growth spurt. Factors fuelling this include an ageing population, advancements in surgical techniques, and innovation in medical materials and implants. These areas have seen a compound annual growth rate (CAGR) forecast that suggests a substantial increase in market value over the next five to ten years, indicating a fertile ground for the commercialisation of Stem Cell Therapy Ltd.’s product lines.
Investment Terms
Regarding Stem Cell Therapy Ltd.’s investment terms, here’s a detailed explanation based on the provided conditions:
- Management Buyout: A transaction involving the sale of management rights.
- Transaction Structure:
- Acquisition of existing major shareholders’ stakes.
-
- New paid-in capital increase
- Expecting a new investor to hold between 30% to over 50% post-transaction.
- Use of Funds: The capital raised from the new rights issue is earmarked for the company’s operational expenses and clinical trials.
- Technical Listing: Stem Cell Therapy Ltd. aims to apply for a technical listing in the Korean public stock market within a year, providing a potential exit opportunity for financial investors through an IPO, should it be successful.
- Strategic Investment:
- Favoured for its potential to enhance company growth with the right infrastructure.
- If a strategic investment occurs, the current CEO wishes to continue as CTO.
Estimated M&A and PMI Strategies
When considering M&A strategies, a nuanced approach is required, especially within the pharmaceutical industry.
- M&A Strategies
- Within the same industry, this acquisition can significantly enhance the product lineup, propelling the buyer’s portfolio through synergistic integration of the target’s offerings.
- A cross-industry acquisition serves as a strategic pivot into new markets, potentially reducing risk through diversification for the buyer.
- Concise PMI and Value-Up Strategies
- The ongoing projects must be evaluated for alignment with the core business focus, potentially leading to the discontinuation of peripheral initiatives.
- Cost efficiencies should be scrutinised through rigorous analysis to identify savings without compromising on the quality of research or workforce morale.
- It is vital to safeguard against the loss of key staff members; their expertise is often irreplaceable and central to the company’s success.
- Enhancing sales in existing ancillary markets, such as the cosmetic ingredients sector, could bolster cash flows and support operational funding.
- The strategy for listing should be meticulously planned to meet the market criteria within the stipulated timeframe, ensuring readiness for the potential IPO.
- A clear roadmap is essential for success in Phase 2 trials, necessitating comprehensive strategic planning.
- The commercialisation plan for M801 should be detailed, setting out each step towards market entry and revenue generation.
Interested in the above companies?
Let’s guide your journey.
Contact us now for expert insights and personalised advisory services on leading companies.